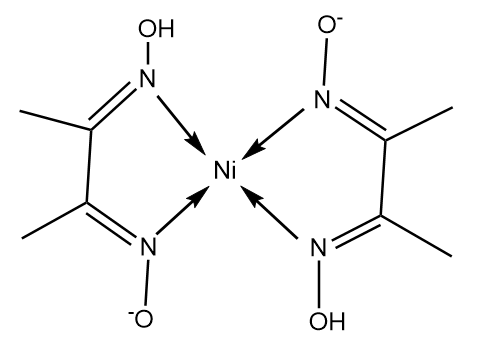
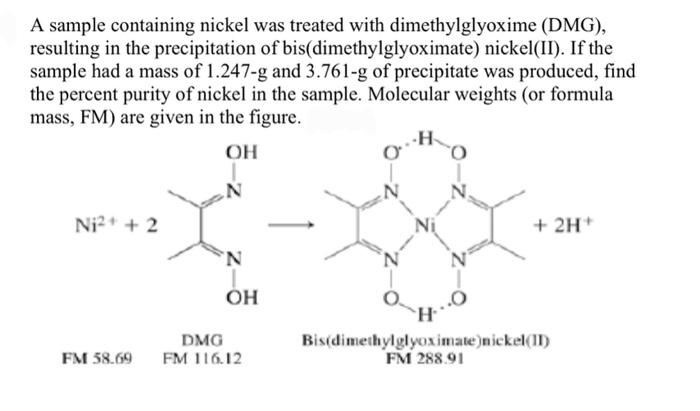
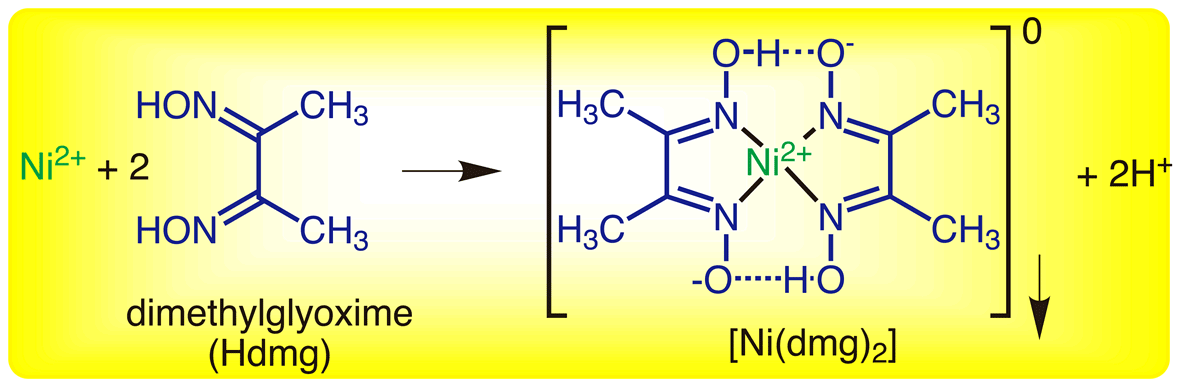
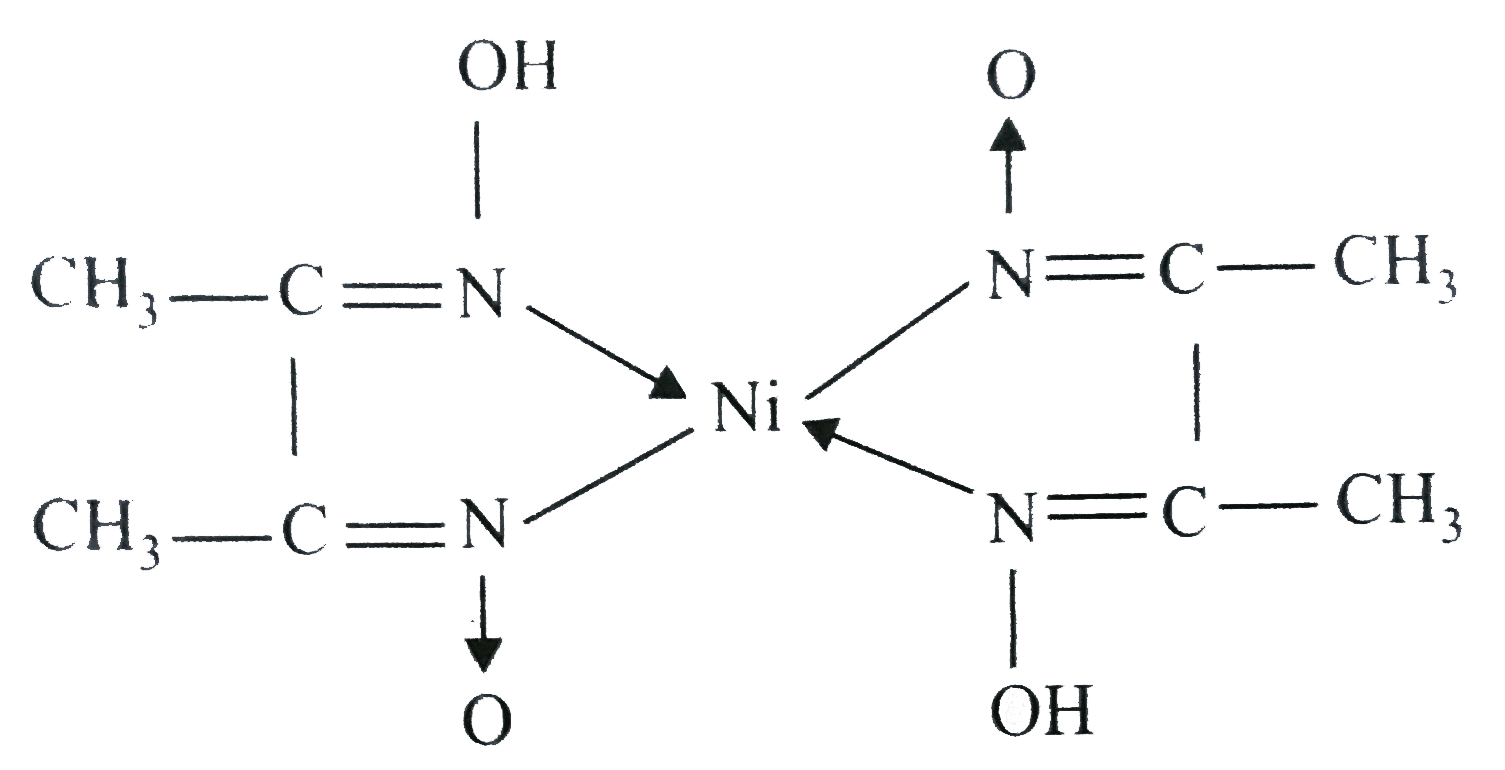
In basic medium the amount of Ni2+ in a solution can be estimated with the dimethylglyoxime reagent. The correct statement(s) about the reaction and the product is(are):

Dimethylglyoxime (C4H8N2O2) - Structure, Molecular Mass, Properties and Uses of Dimethylglyoxime, Dimethylglyoximato Ligand
Ni^2+ can be estimated by used dmg and forms a Rosy red ppt. the complex is extra stabilised by which bonds ?
![Structure of dimethylglyoxime and nickel-dimethylglyoxime complex [179]. | Download Scientific Diagram Structure of dimethylglyoxime and nickel-dimethylglyoxime complex [179]. | Download Scientific Diagram](https://www.researchgate.net/publication/346253195/figure/fig4/AS:1022313969491973@1620750052886/Structure-of-dimethylglyoxime-and-nickel-dimethylglyoxime-complex-179.png)
Structure of dimethylglyoxime and nickel-dimethylglyoxime complex [179]. | Download Scientific Diagram

Ultratrace Detection of Nickel(II) Ions in Water Samples Using Dimethylglyoxime-Doped GQDs as the Induced Metal Complex Nanoparticles by a Resonance Light Scattering Sensor | ACS Omega

Effective vacuum residue upgrading using sacrificial nickel(II) dimethylglyoxime complex in supercritical methanol - ScienceDirect